Imagine Eyes to meet pharmas to speed up the development of therapies
To accelerate the clinical development of new retinal treatments, we propose to implement advanced retinal imaging technology and highly sensitive biomarkers in clinical trials. In November, our team introduces this approach to pharma sponsors, clinical investigators and CROs, in a roadshow in Boston and New York areas.
One-pager download link
New biomarkers for clinical trials
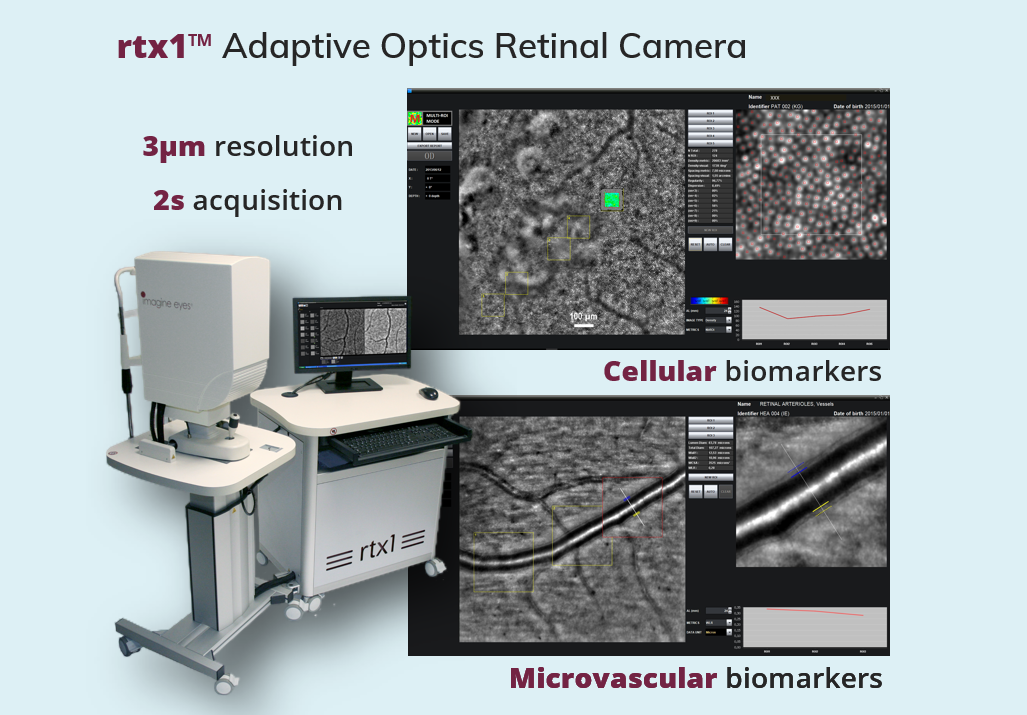
Our rtx1 retinal imaging device provides cellular and microvascular biomarkers. Compared with state-of-the-art techniques, rtx1 biomarkers allow measuring the progression of retinal diseases on timescales that are 5 times shorter, and deliver much earlier signals of treatment safety and effectiveness.
Expected benefits
- Five-fold acceleration of clinical investigations in new retinal therapies
- Multimillion dollar savings in clinical development costs
- New capabilities to further personalize therapies for retinal diseases
Evidence from published studies
Clinical investigations in inherited retinal dystrophy (IRD) and age-related macular degeneration (AMD) have shown that rtx1 detects disease progression in less than 6 months in IRD[1], and less than 1 month in dry AMD[2]. First implementation in therapeutic studies have allowed investigators to confirm the structural stability of transplanted epithelial stem cells in wet AMD[3], and monitor the rescue of photoreceptor cells by gene therapy in IRD[4].
Smooth implementation
Imagine Eyes has supported dozens of clinical investigations that used rtx1 imaging devices. Based on this experience, we offer all the services required for a successful implementation of rtx1 biomarkers in studies and trials of various phases. Our services include imaging protocol design, customer support, device rental and logistics. We partner with Emsere, a leading provider of equipement for clinical trials, to offer a global coverage.
To discuss about your projects, write us at: contact@imagine-eyes.com
About Imagine Eyes
Manufacturer of ophthalmic imaging devices, Imagine Eyes is the leading pioneer in high-resolution retinal imaging. With our products, clinicians examine the back of the eye at the microscopic scale, and exploit superior biomarkers of retinal diseases.
Key figures
- Team of 20 experts in biophotonics and ophthalmic imaging
- Latest funding: € 4.9m grant from the European Commission
- Technology proven by 230 peer-reviewed publications
- Products adopted by 90 clinical investigation centers in 18 countries
About the technology
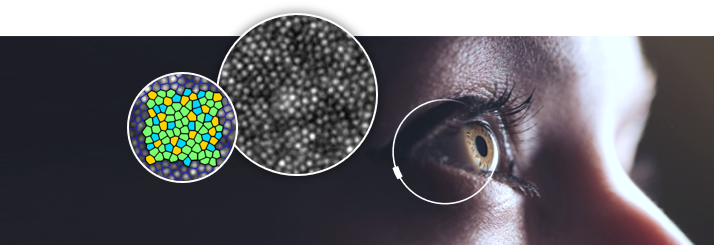
rtx1 delivers highly sensitive biomarkers thanks to adaptive optics (AO) imaging, a technology that enables retinal examinations at the cellular scale. Read more here.
References
[1] Roshandel et al., TVST, 2021, DOI: 10.1167/tvst.10.14.11
[2] Gocho et al., IOVS, 2013, DOI: 10.1167/iovs.12-10672
[3] Takagi et al., Ophthalmol Ret, 2019, DOI: 10.1016/j.oret.2019.04.021
[4] Kortuem et al. Acta Ophthalmol, 2021, DOI: 10.1111/aos.14990
More clinical studies using rtx1 here.
Supported by