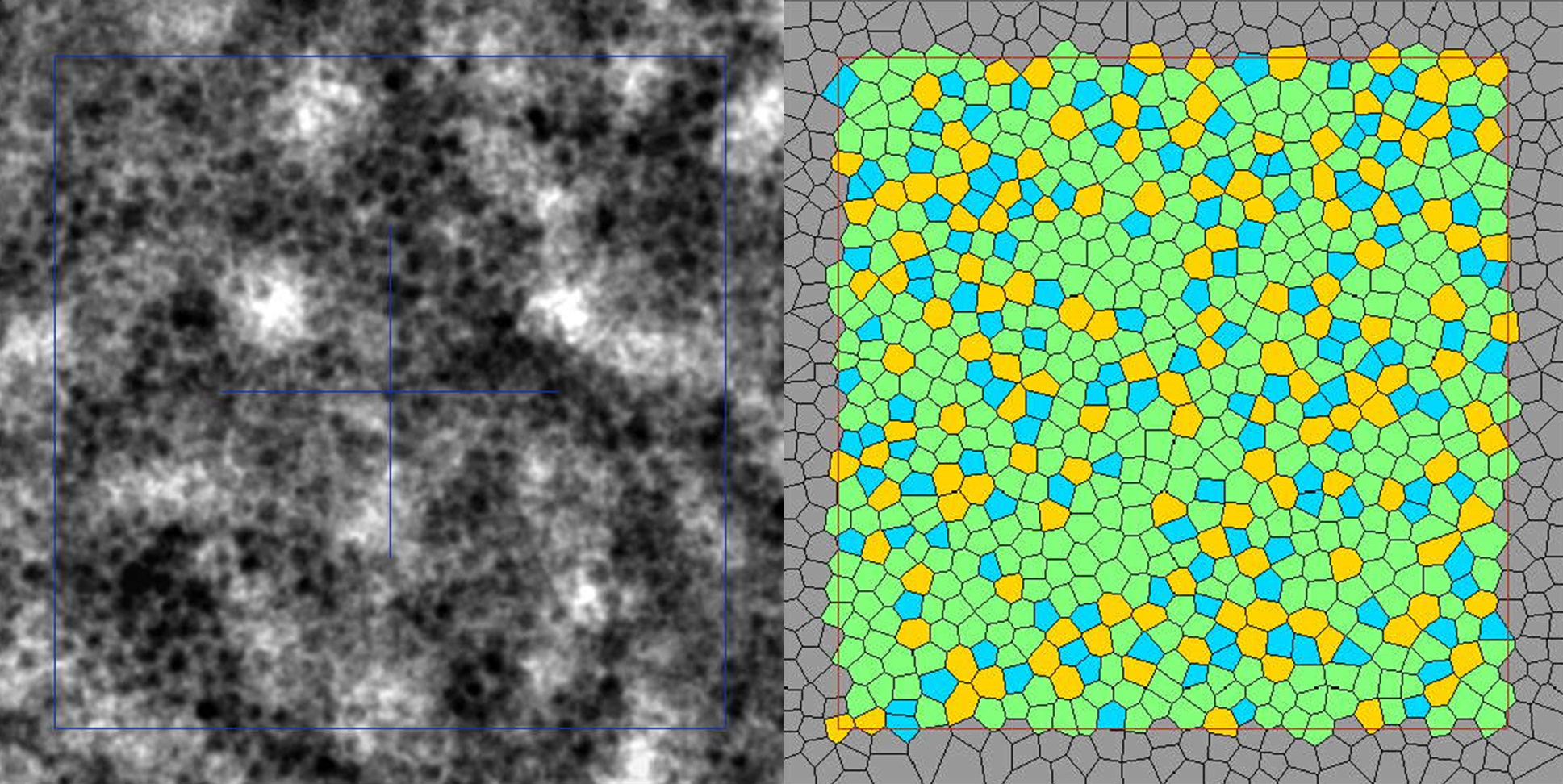
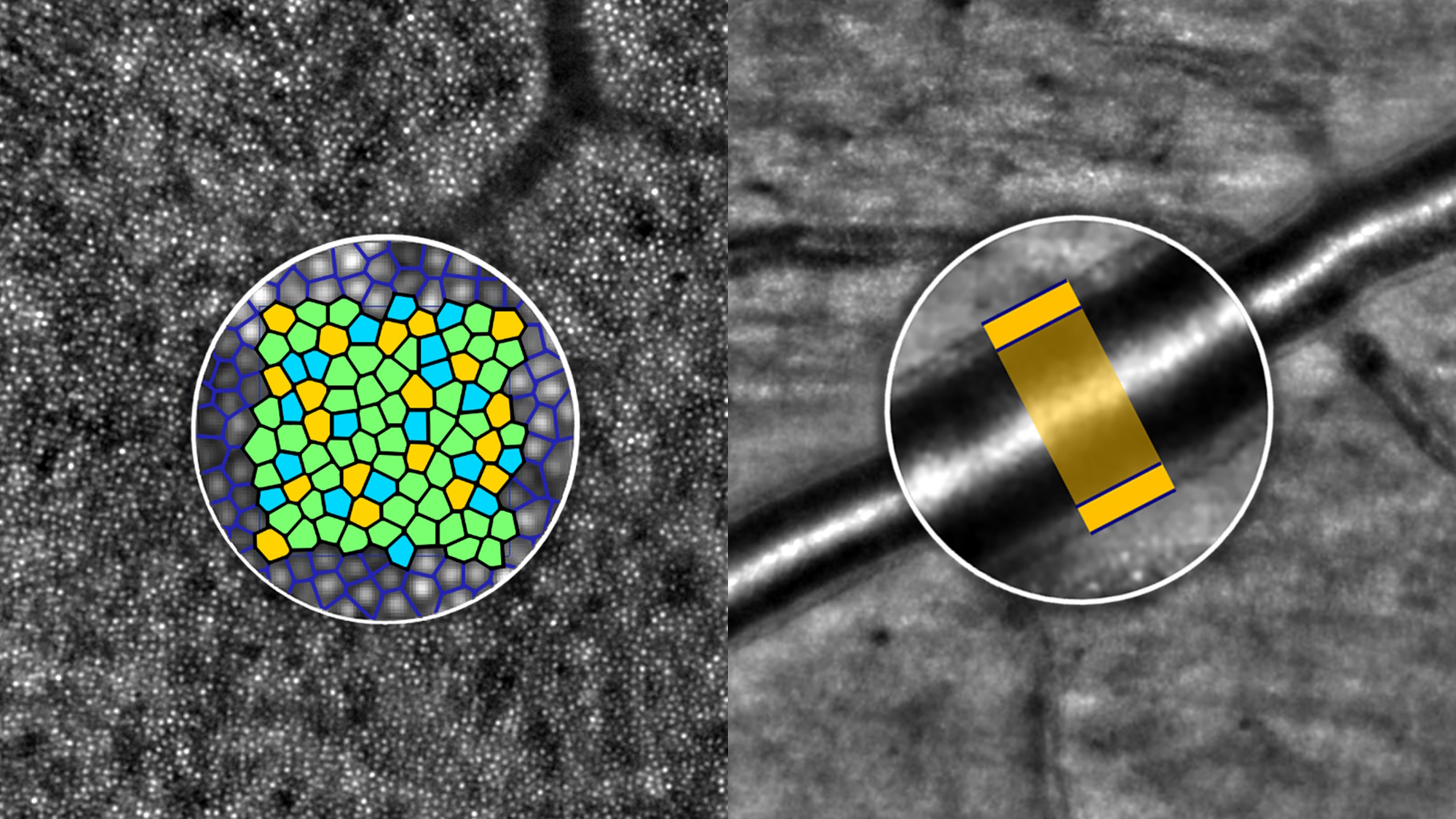
Clinical imaging of the RPE cell mosaic
Gocho et al.1 provide solid evidence for RPE cell imaging with the rtx1-TFI transscleral illumination module, by comparing this new modality with a well-established AF-AOSLO technique.
AMD: prediction of progression to atrophic stages
Shahabi et al.2 characterize AMD phenotypes at the photoreceptor level in 48 eyes with early-onset macular degeneration. Additionally, two follow-up studies address the role of hyporeflective clumps (HRCs) in AMD progression. Paques et al.3 show that HRCs propagate centrifugally – ahead of atrophic area borders – during GA progression. Holmes et al.4 observe an increased clustering of HRCs at baseline in eyes that progress to complete atrophy later in the study. These findings suggest that both the distribution and dynamic properties of HRCs may provide valuable biomarkers in AMD. Furthermore, Paques et al.5 report that rtx1-TFI transscleral imaging improves the identification of these HRCs, enabling more precise phenotyping.
Diabetes: revealing previously undetectable structural and functional changes
Muthiah et al.6 report changes in the photoreceptor mosaic at all stages of diabetic macular ischemia, and associations between these changes and functional findings. Bernardes et al.7 implement and validate a new technique using rtx1 to assess the neurovascular coupling response in diabetic patients.
Inherited retinal diseases: phenotyping at the cellular and microvascular levels
Van Haute et al.8 use rtx1 to characterize the phenotype of MFSD8-related retinal disease, for the first time at the photoreceptor level. Samelska et al.9 find significant differences in microvascular structure between healthy subjects and patients with retinal dystrophies, including Stargardt, cone dystrophy and cone-rod dystrophy. Ota et al.10 elucidate the attenuation of retinal arterioles observed in fundus images in retinitis pigmentosa, by analyzing the vessel lumen and wall structure in rtx1 images.
rtx1 as a tool to assess vitroretinal surgery outcomes
Mihalache et al.11 evaluate photoreceptors with rtx1 in 41 cases of postoperative retinal detachment and show statistically significant associations between cone mosaic metrics and clinical visual outcomes.
Photoreceptor analysis enhanced with AI
Andrade de Jesus et al.12 present a deep-learning model for automated segmentation of photoreceptors in rtx1 images and demonstrate that AI detection outperforms expert human graders.
SCHEDULE AND ROOM NUMBER INFORMATION
[1] Gocho et al. Comparing two adaptive optics imaging methods of retinal pigmented epithelial (RPE) cells
ARVO – Lecture room Tahoma 2
May 6 – 4:00PM-4:15PM
[2] Shahabi et al. Adaptive optics in patients with early-onset macular degeneration
ARVO – Poster A0159
May 6 – 3:00PM-4:45PM
[3] Paques et al. Dynamics of the redistribution of hyperpigmented spots during enlargement of geographic atrophy
ARVO – Poster A0167
May 6 – 3:00PM-4:45PM
[4] Holmes et al. Using adaptive optics to assess hyporeflective clumps as a biomarker for progression to cRORA in the PINNACLE study
ARVO – Lecture room 6E
May 7 – 2:45PM-3:00PM
[5] Paques et al. Combined transpupillary and transscleral adaptive optics imaging in dry age-related maculardegeneration
Imaging in the Eye Conference – Lecture hall Tahoma
May 4 – 3:00PM-3:15PM
[6] Muthiah et al. Adaptive optics (AO) retinal imaging delineates retinal changes in patients at all ETDRS stages of diabetic macular ischemia, undetectable by other structural and functional imaging in mild to moderate ischemia
ARVO – Poster B0530
May 9 – 8:00AM-9:45AM
[7] Bernardes et al. Neurovascular coupling reflex: contralateral hardware stimulation setup and coupling index determination
ARVO – Poster A0313
May 7 – 1:15PM-3:00PM
[8] Van Haute et al. Phenotypic Variability and Evolution of MFSD8-related Retinal Disease
ARVO – Poster B0877
May 9 – 8:00AM-9:45AM
[9] Samelska et al. Blood vessel morphology evaluation in inherited retinal dystrophies
ARVO – Poster A0310
May 7 – 1:15PM-3:00PM
[10] Ota et al. Retinal arterial lumen diameter is reduced and wall thickness is not altered in eyes with retinitis pigmentosa
ARVO – Poster A0351
May 7 – 8:30AM-10:15AM
[11] Mihalache et al. Adaptive Optics Imaging in Retinal Detachment: A Retrospective Analysis
ARVO – Poster B0547
May 5 – 1:00PM-2:45PM
[12] Andrade de Jesus et al. Automated segmentation of cone photoreceptors in AO-FIO: a multi-center study
ARVO – Poster B0644
May 5 – 3:15PM-5:00PM